Italo Tempera, Ph.D.
-
Associate Professor, Gene Expression and Regulation Program, Ellen and Ronald Caplan Cancer Center
-
Associate Director for Cancer Research Career Enhancement, Ellen and Ronald Caplan Cancer Center
Tempera’s research focus is on the epigenetic mechanisms underlying Epstein Barr virus (EBV) infection to identify new viral functions that can be targeted as novel therapeutic approaches for treating EBV-associated cancers.
Tempera obtained his B.Sc. and Ph.D. degrees from University of Rome “La Sapienza”, Italy. He was a postdoctoral fellow at Wistar and established his laboratory at the Fels Institute for Cancer Research and Molecular Biology at Temple University, where he was promoted to associate professor. In 2020, Tempera returned to Wistar as an associate professor in the Gene Expression & Regulation Program.
The Tempera Laboratory

The Tempera Laboratory
Approximately 90 percent of the world population is infected with EBV and carries the virus in a silent state for life. Although EBV infection is asymptomatic in most cases, in some people it can cause infectious mononucleosis. In immunocompromised individuals, such as in HIV-infected persons and transplant recipients, EBV can cause B-cell transformation and malignancies including Burkitt’s lymphoma, nasopharyngeal carcinoma, and Hodgkin’s and non-Hodgkin’s lymphomas. EBV-associated cancers are still treated with chemotherapy, even though they have a specific pathogenic cause, but various attempts are being made to target EBV directly and develop EBV-specific therapies.
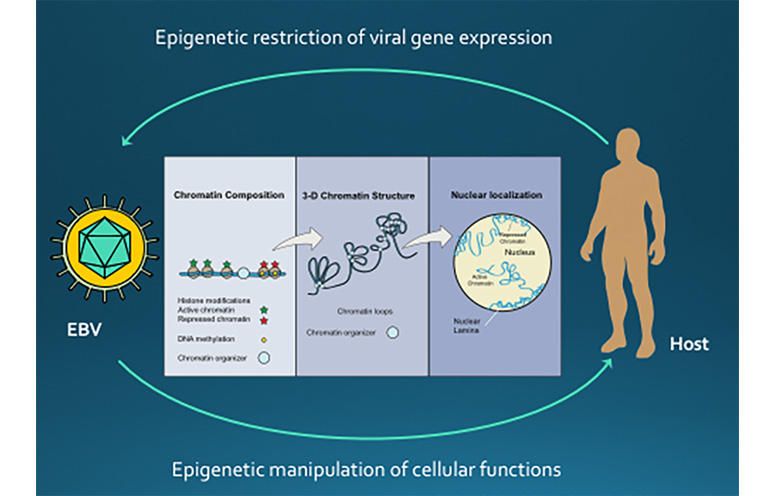
One approach to control EBV infectivity is by changing the expression of viral genes. The Tempera lab studies how epigenetics contributes to regulating the gene expression patterns adopted by EBV during latency. They utilize their expertise in genomics and genome-wide data analysis to better understand the link between the three-dimensional structure of chromosomes, chromatin composition and gene expression during EBV latency.
-
Associate Staff Scientist
Lisa Beatrice Caruso
-
Postdoctoral Fellow
Giorgia Napoletani, Ph.D.
-
Graduate Students
Sarah Johnson
Davide Maestri -
Research Technician
Sarah Boyle
-
Motivated candidates are encouraged to inquire about the position below. Contact itempera@wistar.org.
Postdoctoral Fellow
Learn more about the members of the Tempera Lab
Research
EPIGENETICS AND CTCF IN EBV LATENCY
EBV-induced malignancies have been challenging to target, in large part because EBV establishes a latent infection with complex and dynamic gene expression patterns. These patterns are referred to as “latency types” and can adapt to diverse host cell types and immunological responses. This dynamic gene expression allows EBV to escape eradication by immune surveillance and persist as a long-term, latent infection. In a series of studies, our group has explored the role of epigenetics in the regulation of EBV latency.
Although a role for epigenetic modifications in EBV has been appreciated for decades, our results over the past years have yielded surprising and important insights into its mechanism. We found that different EBV latency programs correlate with different epigenetic states of the viral episome, and we identified CTCF as the primary cellular factor that regulates these epigenetic patterns. Overall our work shows that:
- The epigenetic regulation of EBV is a dynamic process involving both histone modifications and DNA methylation;
- The CTCF cellular factor is critical for the maintenance of the viral chromatin state of EBV;
- CTCF regulates the viral promoters.
These findings defined the important function of CTCF as a chromatin organizer of the EBV viral genome.

EBV GENOME ARCHITECTURE
Our team has explored the three-dimensional organization of the EBV genome during latency. Since CTCF promotes chromatin loops in the human genome, we tested the possibility that CTCF acts in a similar way on the EBV genome. By combining 3C assay, ChIP-seq and EBV genetic methods, our group has shown that:
- The EBV genome adopts alternative chromosome conformations during latency;
- CTCF is the critical cellular factor that mediates chromatin loop formation in EBV;
- The viral enhancer at the Ori P region of EBV serves as a “chromatin hub” regulating viral promoters through chromatin loops;
- The disruption of EBV three-dimensional organization impairs viral gene expression.
Our results have important biological implications since they demonstrate for the first time that viral genomes can also regulate their function by adopting different three-dimensional configurations.

PARP1 AND EBV INFECTION
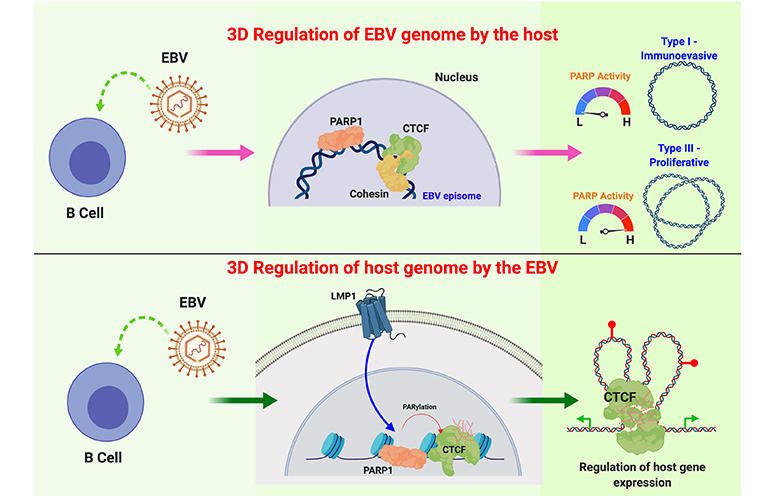
We discovered that PARP1 can interact with the EBV genome. PARP1 is a host enzyme that post-translationally modifies proteins through the transfer of ADP-ribose from NAD+ onto acceptor proteins. The role of PARP1 in DNA damage and apoptosis is well characterized; however, in recent years, PARP1 has also been implicated in chromatin modification, transcriptional regulation and inflammation.
Evolutionary analyses have identified other PARP family proteins that likely have evolved a role in host-virus conflicts. Indeed, a body of evidence suggests that PARP1 may serve as a stress sensor — a function that would be especially relevant in regulating herpesvirus lytic reactivation. CTCF is also PARylated by PARP1, subsequently altering its insulator function.
Based on the established role of CTCF in EBV latency, the functional interactions of PARP1 and CTCF, and because CTCF and PARP1 are both implicated as viral restriction factors in other herpesviruses, our group has studied the role of PARP1 in regulating the EBV epigenome.
PARP1 AND EBV-INDUCED B CELL TRANSFORMATION
We have shown for the first time that LMP1 signaling affects important chromatin-modifying enzymes, specifically PARP1. PARP1 regulates global gene expression by relaxing the chromatin structure and inhibiting the accumulation of repressive histone marks.
We discovered that inhibiting PARP1 suppresses malignant transformation in vitro and represses the expression of previously identified LMP1 genetic targets. Thus, our team has explored the hypothesis that PARP1 activity plays an important role in EBV-induced oncogenesis. Current projects in our laboratory aim at establishing PARP inhibitors as a novel target for EBV-induced cancers and determining the pathogenic mechanisms involved in EBV-mediated tumor initiation.

PARP1 AND EZH2 FUNCTIONS
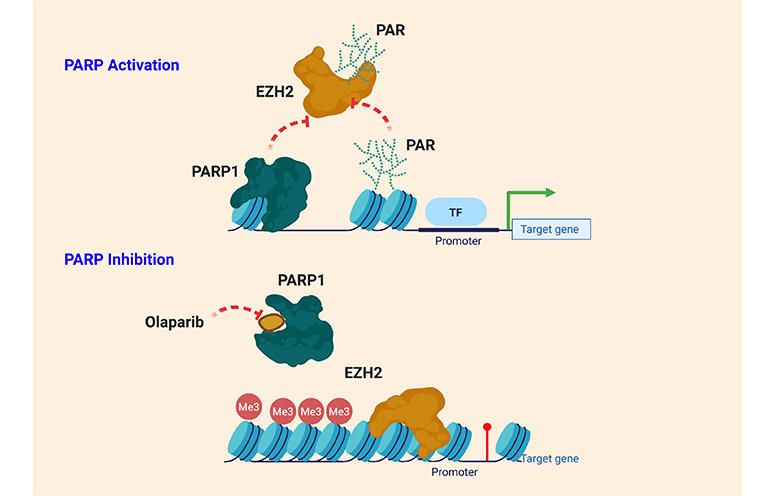
Our group discovered that inhibition of PARP1 activity dramatically changes the expression levels of hundreds of genes, including genes involved in cancer. We found that increased levels of the Polycomb Repressive Complex 2 (PRC2) catalytic subunit EZH2 are responsible for some effects caused by PARP inhibition.
We reported for the first time that:
- PARP1 and EZH2 occupancy negatively correlate across the genome;
- PARP1 can directly modify EZH2;
- PARylation alters the enzymatic activity of EZH2.
Based on these data, our team has explored the hypothesis that PARP1 and PARylation play an important and underappreciated role in EZH2 activity and inhibitors of PARP can alter PRC2-mediated gene repression. We are working to establish PARP1 and PARylation as a novel mechanism of EZH2 regulation and determine mechanisms and functional relevance of PARP-mediated EZH2 inhibition.
Tempera Lab in the News
Selected Publications
Poly(ADP-ribose) polymerase 1 is necessary for coactivating hypoxia-inducible factor-1-dependent gene expression by Epstein-Barr virus latent membrane protein 1.
Hulse, M., Caruso, L.B., Madzo, J., Tan, Y., Johnson, S., Tempera, I. “Poly(ADP-ribose) polymerase 1 is necessary for coactivating hypoxia-inducible factor-1-dependent gene expression by Epstein-Barr virus latent membrane protein 1.” PLoS Pathog. 2018 Nov 5;14(11):e1007394. doi: 10.1371/journal.ppat.1007394.
PARP1 Stabilizes CTCF Binding and Chromatin Structure To Maintain Epstein-Barr Virus Latency Type.
Lupey-Green, L.N., Caruso, L.B., Madzo, J., Martin, K.A., Tan, Y., Hulse, M., Tempera, I. “PARP1 Stabilizes CTCF Binding and Chromatin Structure To Maintain Epstein-Barr Virus Latency Type.” J Virol. 2018 Sep 15; 92(18): e00755-18. Prepublished online 2018 Jul 5. Published online 2018 Aug 29. doi: 10.1128/JVI.00755-18.
Poly(ADP-ribose) Polymerase 1, PARP1, modifies EZH2 and inhibits EZH2 histone methyltransferase activity after DNA damage.
Caruso, L.B., Martin, K.A., Lauretti, E., Hulse, M., Siciliano, M., Lupey-Green, L.N., Abraham, A., Skorski, T., Tempera, I. “Poly(ADP-ribose) Polymerase 1, PARP1, modifies EZH2 and inhibits EZH2 histone methyltransferase activity after DNA damage.” Oncotarget. 2018 Feb 13; 9(12): 10585–10605. Published online 2018 Jan 24. doi: 10.18632/oncotarget.24291.
PARP1 restricts Epstein Barr Virus lytic reactivation by binding the BZLF1 promoter.
Lupey-Green, L.N., Moquin, S.A., Martin, K.A., McDevitt, S.M., Hulse, M., Caruso, L.B., Pomerantz, R.T., Miranda, J.L., Tempera, I. “PARP1 restricts Epstein Barr Virus lytic reactivation by binding the BZLF1 promoter.” Virology. 2017 Jul;507:220-230. Published online 2017 Apr 26. doi: 10.1016/j.virol.2017.04.006.
Epstein-Barr Virus Oncoprotein LMP1 Mediates Epigenetic Changes in Host Gene Expression through PARP1.
Martin, K.A., Lupey, L.N., Tempera, I. “Epstein-Barr Virus Oncoprotein LMP1 Mediates Epigenetic Changes in Host Gene Expression through PARP1.” J Virol. 2016 Oct 1; 90(19): 8520–8530. Prepublished online 2016 Jul 20. Published online 2016 Sep 12. doi: 10.1128/JVI.01180-16.


