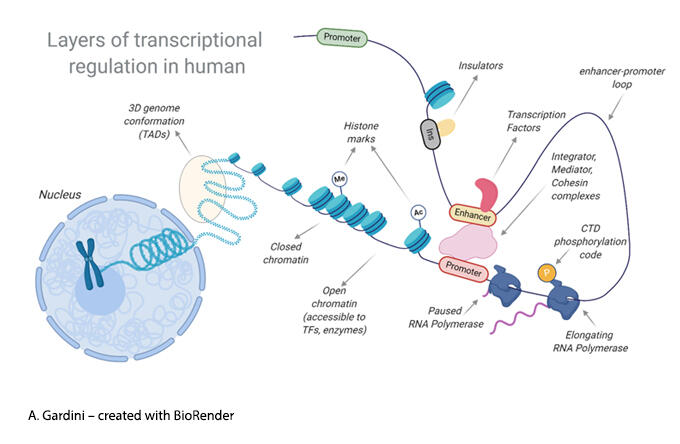
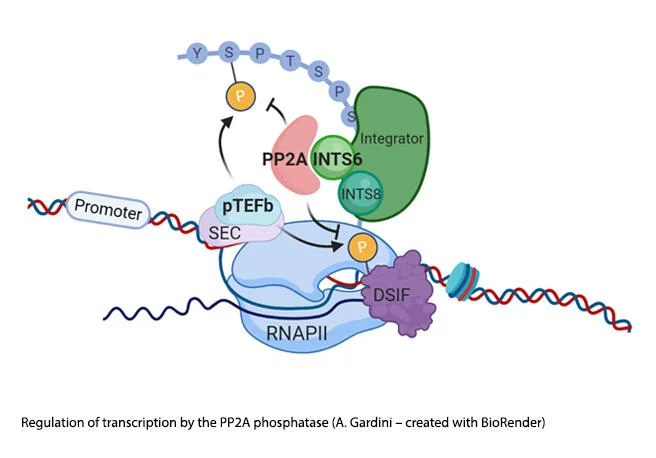
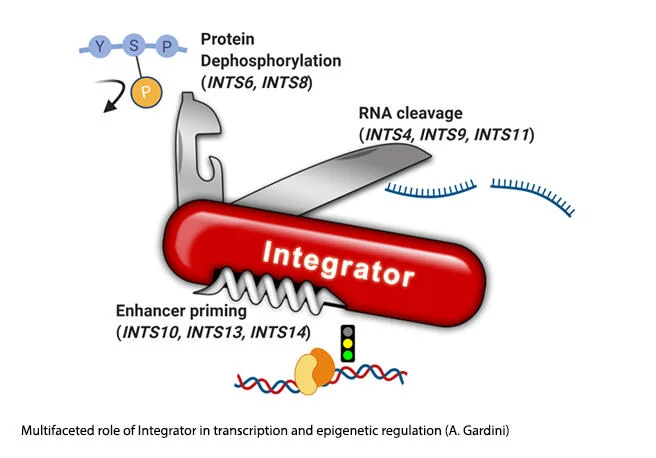
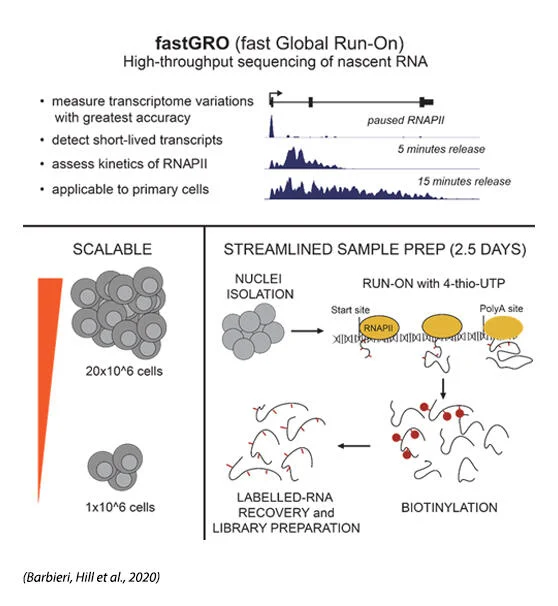
Joseph Salvino, Ph.D.
-
Professor, Molecular and Cellular Oncogenesis Program, Ellen and Ronald Caplan Cancer Center
-
Scientific Director, Molecular Screening & Protein Expression Facility
Salvino is the first medicinal chemist to join Wistar’s staff, and his work to identify novel small molecule lead compounds provides a strong basis for collaboration with all Wistar researchers.
Salvino has more than 30 years of experience in drug discovery, including early stage hit-to-lead, lead optimization, and preclinical development where several drug candidates have successfully completed human clinical trials, including Entereg®, ADL-101, and Radezolid®. Prior to joining Wistar, Salvino was a professor in the Department of Pharmacology and Physiology at Drexel University College of Medicine. Before that, he held high level positions including vice president, senior director, and director in the biotechnology and pharmaceutical industries of Sterling Winthrop, Rhone-Poulenc Rorer, Aventis, Rib-X Pharmaceuticals, Adolor Corporation, and Cephalon.
Salvino received his Ph.D. in organic chemistry from Brown University and completed postdoctoral training in synthetic and medicinal chemistry at the University of Pennsylvania under the mentorship of K.C. Nicolaou and Ralph F. Hirschmann.
The Salvino Laboratory

The Salvino Laboratory
Salvino’s research is directed at drug discovery and development. His laboratory focuses on early drug discovery and small molecule tool compounds for in vivo target validation to confirm the pharmacological relevance and “drugability” of a therapeutic target. His lab’s work in medicinal chemistry optimization is focused on the design and creation of new chemical analogs to understand structure activity relationships (SAR) critical for optimizing potency and in vivo efficacy for a lead series. This effort is important to help identify a potential preclinical drug candidate or future therapeutic agent against a new biological target.
Salvino is the Scientific Director of the Molecular Screening & Protein Expression Shared Resource which closely aligns screening capabilities for lead optimization and hit-to-lead activities that are ongoing in his laboratory.
Current projects include compounds blocking HIV and other antivirals, glutamate transporter (EAAT2) stimulators, ACSS2 Inhibitors, Proteolysis Targeting Chimeras (PROTACs) with a focus on CDK6, Mdm2, and Tert degraders, small molecules targeting protein-protein interactions, chemical probes for assay development, and cancer targeting and gram-negative bacteria targeting pro-drugs.
-
Postdoctoral Fellows
Adi Narayana Reddy Poli, Ph.D.
Jitendra Gour, Ph.D.
Irfan Khan, Ph.D.
Nitesh Nandwana, Ph.D.. -
UPenn Capstone Masters in Chemistry Students
Heyang Shen
Honey Shah
Salvino Lab in the News
Selected Publications
-
Targeting the CDK6 Dependence of Ph+ Acute Lymphoblastic Leukemia.
Porazzi, P., Dominici, M.D., Salvino, J., Calabretta, B. “Targeting the CDK6 Dependence of Ph+ Acute Lymphoblastic Leukemia.” Genes (Basel). 2021 Aug 29;12(9):1355. doi: 10.3390/genes12091355.
-
Metabolic Control of Brisc-shmt2 Assembly Regulates Immune Signalling.
Walden, M., Tian, L., Ross, R.L., Sykora, U.M., Byrne, D.P., Hesketh, E.L., Masandi, S.K., Cassel, J., George, R., Ault, J.R., Oualid, F.E., et al. “Metabolic Control of Brisc-shmt2 Assembly Regulates Immune Signalling.” Nature. 2019 Jun;570(7760):194-199. doi: 10.1038/s41586-019-1232-1. Epub 2019 May 29.
-
Targeting ACSS2 with a Transition-State Mimetic Inhibits Triple-Negative Breast Cancer Growth.
Miller, K.D., Pniewski, K., Perry, C.E., Papp, S.B., Shaffer, J.D., Velasco-Silva, J.N., Casciano, J.C., Aramburu, T.M., Srikanth, Y.V.V., Cassel, J., et al. “Targeting ACSS2 with a Transition-State Mimetic Inhibits Triple-Negative Breast Cancer Growth.” Cancer Res. 2021 Mar 1;81(5):1252-1264. doi: 10.1158/0008-5472.CAN-20-1847. Epub 2021 Jan 7.
-
Selective inhibition of Ph-positive ALL cell growth through kinase-dependent and -independent effects by CDK6-specific PROTACs.
Dominici, M.D., Porazzi, P., Xiao, Y., Chao, A., Tang, H., Kumar, G., Fortina, P., Spinelli, O., Rambaldi, A., Peterson, L.F., et al. “Selective inhibition of Ph-positive ALL cell growth through kinase-dependent and -independent effects by CDK6-specific PROTACs.” Blood. 2020 Apr 30;135(18):1560-1573. doi: 10.1182/blood.2019003604.
-
Development of a Novel Inducer for Ebv Lytic Therapy.
Tikhmyanova, N., Paparoidamis, N., Romero-Masters, J., Feng, X., Mohammed, F.S., Narayana Reddy, P.A., Kenney, S.C., Lieberman, P.M., Salvino, J.M. “Development of a Novel Inducer for Ebv Lytic Therapy.” Bioorg Med Chem Lett. 2019 Aug 15;29(16):2259-2264. doi: 10.1016/j.bmcl.2019.06.034. Epub 2019 Jun 22.