Amelia Escolano, Ph.D.
-
Assistant Professor, Vaccine & Immunotherapy Center
The Escolano Lab investigates novel vaccination strategies against highly mutating viruses.
We are interested in understanding the unique features of the humoral and cellular immune responses to sequential immunization, with special focus on the process of antibody affinity maturation in the germinal centers. Our goal is to rationally design vaccination approaches to induce potent and long-lasting antibody responses against pathogens that diversify over time.
Dr. Amelia Escolano is an Assistant Professor in the Vaccine & Immunotherapy Center and a Wistar Institute Assistant Professor in Microbiology at the University of Pennsylvania.
Escolano obtained her BS degree in Biochemistry from the University of Oviedo, Spain and a master’s degree from Centro de Biologia Molecular Severo Ochoa in Madrid, Spain. She received additional training at the University of Turku, Finland and the Genome Research Institute in Cincinnati, Ohio. Escolano obtained her PhD in biochemistry and molecular biology from Autonoma University of Madrid after completing her pre doctoral studies at the Spanish National Center for Cardiovascular Research (CNIC) in Madrid. She trained as a postdoctoral fellow in the laboratory of Michel Nussenzweig at The Rockefeller University in New York and joined The Wistar Institute as an Assistant Professor in 2021. Escolano is a Pew Biomedical Scholar, a recipient of the regional Blavatnik award for young scientists (finalist) and has been recognized with a NIH Director’s New Innovator Award (DP2).
The Escolano Laboratory

The Escolano Laboratory
Viruses such as HIV-1, influenza and SARS-CoV-2 have the ability to rapidly mutate as a mechanism to escape from the host immune system. As a result, highly mutating viruses are largely diverse, with multiple different circulating variants characterized by individual antigenic and infectivity properties. The design of efficacious vaccines against highly diverse viruses is extraordinarily challenging. Despite decades of research, no universal vaccines exist against HIV-1 or influenza. Unfortunately, common vaccination strategies cannot elicit the type of broadly neutralizing antibodies required to confer broad protection against these viruses, and some of the currently available vaccines fail to induce long-lasting protection. Consequently, boost immunizations are advised in order to achieve protection.
The Escolano Lab investigates the use of sequential immunization as a novel vaccination strategy aiming to induce broadly neutralizing antibodies against highly diverse viruses. Using HIV-1 as a model virus, state-of-the-art technologies and animal models including wild type mice, transgenic mice and rhesus macaques, the laboratory studies the humoral and cellular immune responses to sequential immunization. The research goal is to identify guidelines for the design of vaccination approaches to induce long-lasting protection against highly mutating viruses in humans.
-
Postdoctoral Fellows
Ignacio Rodriguez Relaño, Ph.D.
Maria Belen Palacio, Ph.D.
Marta Tarquis, Ph.D. -
Graduate Students
Ashwin Skelly
Austin Kriews -
Research Assistant
Caroline Boroughs
Sowmya Meka
Maggie Kerwin
-
Postdoctoral fellow and research assistant positions are available in the Escolano laboratory. Interested applicants are encouraged to contact aescolano@wistar.org.
Research
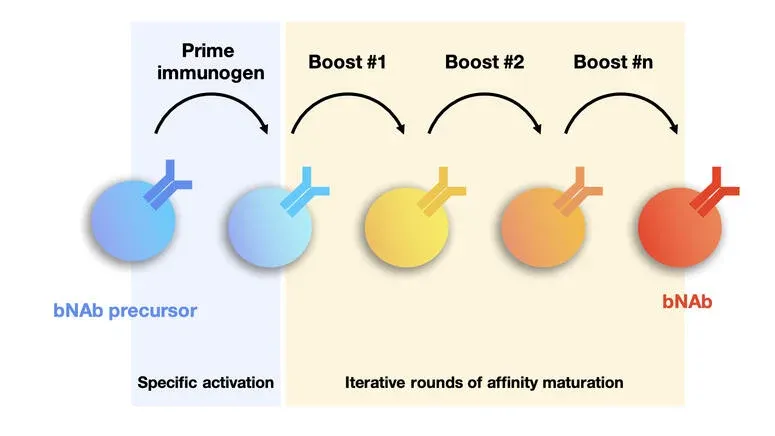
An efficacious antibody-based vaccine against HIV-1 should elicit broadly neutralizing antibodies (bNAbs) targeting conserved epitopes of its envelope (Env) protein. Our previous work showed that common vaccination strategies using repeated boost immunization with the same Env immunogen could not elicit bNAbs. Instead, a new form of vaccination involving sequential immunization was required to elicit highly mutated anti-HIV-1 bNAbs. The reported sequential immunization protocol involved prime immunization with an engineered Env immunogen followed by a series of four additional immunizations with Env immunogens that gradually resembled the native-looking Env. This immunization protocol elicited bNAbs in an immunoglobulin knock-in mouse model with a monoclonal B cell repertoire engineered to carry the inferred germline precursor of a human bNAb (Escolano et al, Cell, 2016). These immunization experiments were the first showing that anti-HIV-1 bNAbs can be elicited by vaccination, and fueled subsequent vaccine design studies. However, despite this significant achievement, no vaccination protocols have been reported that are able to elicit protective levels of bNAbs in wild type organisms with a polyclonal B cell repertoire.
Wild type organisms mount polyclonal antibody responses when encountering complex antigens such as the HIV-1 Env protein. A predominant component of antibody responses elicited by Env immunogens are antibodies to non-conserved or strain-specific epitopes of Env, with no potential to broadly neutralize HIV-1. These antibodies significantly interfere with the development of bNAbs.
The Escolano lab investigates the humoral and cellular immune responses to sequential immunization to establish guidelines for vaccine design. We design and evaluate immunogens and sequential immunization strategies to elicit anti HIV-1 broadly neutralizing antibodies in wild type organisms. Our specific goal is to devise approaches to modulate the immunodominance properties of vaccine candidates aiming to focus the antibody responses to the conserved, neutralization-sensitive epitopes of Env.
Using state-of-the-art technologies for single cell analysis and different animal models including wild type mice, rhesus macaques and a series of recently generated immunoglobulin knock-in and reporter mice, we investigate the process of antibody maturation and the evolution of the different immune compartments upon sequential immunization.
Our laboratory has vast experience designing strategies to isolate antigen-specific B cells from wild type mice, humanized mice and non-human primates, and cloning their antibody genes.
Antibody cloning from single B cells is an essential tool for characterizing humoral immune responses and obtaining valuable therapeutic and analytical reagents. Antibody cloning from individuals with high serologic titers to HIV-1, influenza, malaria, ZIKV, and SARS-CoV-2 has led to new insights that inform vaccine design efforts.
We have designed cost-effective protocols to identify and purify single antigen-specific B cells, and subsequently clone and produce monoclonal antibodies.
We are using the newly developed methods to isolate and characterize HIV-1-specific B cells from naïve and immunized mice, from vaccinated or simian-human immunodeficiency virus (SHIV)-infected rhesus macaques and from humans. Remarkably, using our approach, we have isolated one of the first anti-HIV-1 bNAbs from a SHIV-infected rhesus macaque, validating the use of macaques as preclinical models for HIV-1 vaccination studies.
The Escolano laboratory uses this state-of-the-art methodology to isolate antibodies against bacteria, tumor neoantigens and viruses, including cancer-associated viruses. Characterization of the isolated antibodies is providing very valuable information to guide vaccine design efforts and the development of new preventative and therapeutic approaches.
The use of animal models in biomedical research has been crucial to investigate the mechanisms of human pathophysiology, evaluate candidate interventions and predict treatment outcomes in humans. Animal Models have been extensively used by the scientific community to examine the cellular and humoral responses to infection and vaccination. Unfortunately, none of these models faithfully recapitulates the setting of a human immune response.
Humanized mice genetically engineered to recapitulate different aspects of the human humoral and/or cellular immune response are highly desirable. In particular, human immunoglobulin knock-in mice (Ig KI mice) are remarkably valuable for vaccine development and drug discovery, as well as for basic immunology studies related to the analysis of B and T cell responses to infection, vaccination, autoimmunity, or cancer. However, current methods to produce Ig KI mice are inefficient, labor-intensive and require special equipment and expertise to perform zygote microinjections.
We have developed a novel technology to efficiently and more easily generate monoclonal Ig KI mice using CRISPR/Cas9. The new technology remarkably simplifies the mouse production process, increases knock-in efficiency and reduces breeding time, thus accelerating the production process and reducing cost.
We are currently using the new technology for high-throughput production of Ig KI mice carrying anti-HIV-1 antibodies, which we use for vaccine design purposes.
Interestingly, this methodology can be adapted to introduce other genetic modifications in the mouse genome including gene insertions and deletions, thus being of great value to other scientific disciplines. The new technology is especially valuable for engineering events involving insertions of long DNA fragments or genetic modification of more than one locus.
-
Postdoctoral Fellows
Ignacio Rodriguez Relaño, Ph.D.
Maria Belen Palacio, Ph.D.
Marta Tarquis, Ph.D. -
Graduate Students
Ashwin Skelly
Austin Kriews -
Research Assistant
Caroline Boroughs
Sowmya Meka
Maggie Kerwin
-
Postdoctoral fellow and research assistant positions are available in the Escolano laboratory. Interested applicants are encouraged to contact aescolano@wistar.org.
Selected Publications
Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice.
Escolano, A., Steichen, J.M., Dosenovic, P., Kulp, D.W., Golijanin, J., Sok, D., Freund, N.T., Gitlin, A.D. Oliveira, T. Araki, T., et al. “Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice.” Cell. 2016 Sep 8;166(6):1445-1458.e12. doi: 10.1016/j.cell.2016.07.030.
Immunization Expands B Cells Specific To HIV-1 V3 Glycan In Mice And Macaques.
Escolano, A., Gristick, H.B., Abernathy, M.E., Merkenschlager, J., Gautam, R., Oliveira, T.Y., Pai, J., West Jr, A.P., Barnes, C.O., Cohen, A.A., et al. “Immunization Expands B Cells Specific To HIV-1 V3 Glycan In Mice And Macaques.” Nature. 2019 Jun;570(7762):468-473. doi: 10.1038/s41586-019-1250-z. Epub 2019 May 29.
Sequential immunization of macaques elicits heterologous, neutralizing antibodies targeting the V3-glycan patch of HIV-1 Env.
Escolano A, Gristick HB, Gautam R, DeLaitsch AT, Abernathy ME, Yang Z, Wang H, Hoffmann MAG, Nishimura Y, Wang Z, Koranda N, Kakutani LM, Gao H, Gnanapragasam PNP, Raina H, Gazumyan A, Cipolla M, Oliveira TY, Ramos V, Irvine DJ, Silva M, West AP Jr, Keeffe JR, Barnes CO, Seaman MS, Nussenzweig MC, Martin MA, Bjorkman PJ. Sequential immunization of macaques elicits heterologous neutralizing antibodies targeting the V3-glycan patch of HIV-1 Env. Sci Transl Med. 2021 Nov 24;13(621):eabk1533. doi: 10.1126/scitranslmed.abk1533. Epub 2021 Nov 24. PMID: 34818054; PMCID: PMC8932345.
A Broadly Neutralizing Macaque Monoclonal Antibody Against The HIV-1 V3-Glycan Patch.
Wang, Z., Barnes, C.O., Gautam, R., Cetrulo Lorenzi, J.C., Mayer, C.T., Oliveira, T.Y., Ramos, V., Cipolla, M., Gordon, K.M., Gristick, H.B., et al. “A Broadly Neutralizing Macaque Monoclonal Antibody Against The HIV-1 V3-Glycan Patch.” Elife. 2020 Oct 21;9:e61991. doi: 10.7554/eLife.61991.
Progress Toward Active Or Passive HIV-1 Vaccination.
Escolano, A., Dosenovic, P., Nussenzweig , M.C. “Progress Toward Active Or Passive HIV-1 Vaccination.” J Exp Med. 2017 Jan;214(1):3-16. doi: 10.1084/jem.20161765. Epub 2016 Dec 21.


